Aim
To demonstrate the process of osmosis in potato cells and observe the movement of water molecules across a semi-permeable membrane.
Materials Required
- Potato
- Water
- Concentrated sugar solution
- Knife
- Spoon
- Beaker (2)
- Measuring scale (optional)
Image Reference
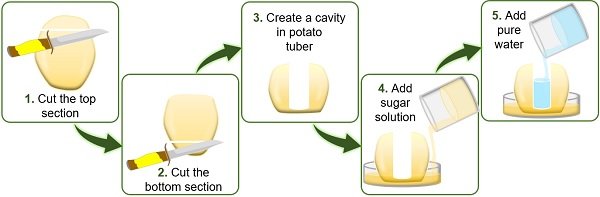
Procedure
- Cut a potato into two equal halves. Carve a small hollow cavity in the center of each half using a knife or spoon.
- Label the two beakers as *A* and *B*. Fill beaker A with plain water and beaker B with a concentrated sugar solution.
- Place one potato half in each beaker, ensuring the cavity faces upwards.
- Add water to the cavity of the potato in beaker A and sugar solution to the cavity of the potato in beaker B.
- Leave the setup undisturbed for 3-4 hours and observe the changes in the level of liquid in the cavities and the size of the potato halves.
Observation
After a few hours:
- The cavity in the potato placed in water (beaker A) remains the same or may fill slightly due to water entering the cells.
- The cavity in the potato placed in sugar solution (beaker B) shows a reduction in liquid as water moves out of the potato cells into the sugar solution.
- The potato in beaker B shrinks slightly due to the loss of water, while the one in beaker A appears firm and turgid.
Inference
This experiment demonstrates the process of osmosis:
- Osmosis is the movement of water molecules from a region of higher water concentration to a region of lower water concentration through a semi-permeable membrane.
- In beaker A, water enters the potato cells due to osmosis, making them turgid.
- In beaker B, water leaves the potato cells into the sugar solution, causing the potato to shrink (plasmolysis).
Precautions
- Ensure the potato slices are of similar size to observe clear differences in the results.
- Handle the knife carefully to avoid injury while carving the potato.
- Use fresh and clean water to avoid contamination affecting the results.
- Leave the setup undisturbed during the experiment for accurate observations.