Aim
To observe and understand the neutralization reaction between an acid and a base, and to learn how an indicator helps in detecting the endpoint of the reaction. The goal is to see how the acid reacts with the base to form salt and water.
Materials Required
- Hydrochloric acid (HCl)
- Sodium hydroxide (NaOH)
- Phenolphthalein solution (indicator)
- Beaker
- Burette
- Stirring rod
Image Reference
Procedure
- Take a few drops of sodium hydroxide (NaOH) solution in a clean beaker. Sodium hydroxide is a strong base and will provide a visible reaction with hydrochloric acid.
- Add a few drops of phenolphthalein indicator to the NaOH solution. The phenolphthalein will turn pink, indicating the alkaline nature of NaOH.
- Fill a burette with hydrochloric acid (HCl) solution. This acid will neutralize the base during the experiment.
- Slowly add hydrochloric acid (HCl) from the burette to the NaOH solution while stirring gently with a stirring rod. This step should be done carefully, drop by drop, to observe the neutralization process clearly.
- Continue adding hydrochloric acid until the pink color of the solution fades and the solution becomes colorless. This indicates that the acid has completely neutralized the base and the neutralization reaction is complete.
Observation
During the experiment, as hydrochloric acid (HCl) neutralizes sodium hydroxide (NaOH), the color of the solution changes from pink to colorless. This color change is an indication that the reaction has reached its neutralization point, where the base has been completely neutralized by the acid, resulting in a neutral solution of water and salt.
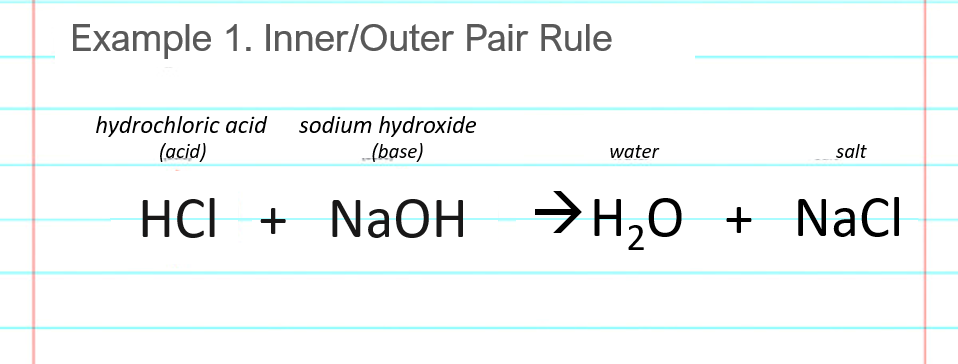
Reaction Equation
NaOH + HCl → NaCl + H2O
This is the general equation for the neutralization reaction between a strong acid (HCl) and a strong base (NaOH), resulting in the formation of salt (sodium chloride) and water.
Precautions
- Handle acids and bases with care as they are corrosive and can cause skin burns. Always wear protective gear like gloves and safety goggles.
- Ensure that the burette is clean before use to avoid any contamination of the chemicals.
- Perform the experiment slowly and carefully, adding hydrochloric acid drop by drop to avoid overshooting the neutralization point.
- Do not dispose of the chemicals directly; follow proper disposal guidelines for acid-base solutions.