Aim
To prepare oxygen gas by decomposing hydrogen peroxide (H₂O₂) using manganese dioxide (MnO₂) as a catalyst, and to observe the characteristics of oxygen gas.
Materials Required
- Hydrogen Peroxide (H₂O₂)
- Manganese Dioxide (MnO₂) (as a catalyst)
- Conical flask
- Delivery tube
- Water trough
- Glowing splint (for testing oxygen)
Image Reference
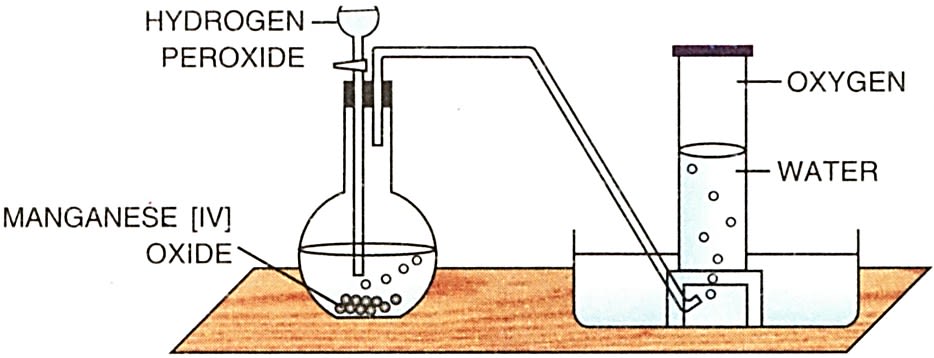
Procedure
- Take a clean conical flask and add a small amount of manganese dioxide (MnO₂) as a catalyst. Manganese dioxide will speed up the decomposition of hydrogen peroxide without being consumed in the reaction.
- Pour hydrogen peroxide (H₂O₂) into the flask. The hydrogen peroxide will start decomposing into water and oxygen gas when the catalyst is added.
- Immediately connect a delivery tube to the flask. The other end of the delivery tube should be immersed in a water trough to collect the evolved gas by upward displacement of water.
- Observe the bubbling and release of oxygen gas as the reaction proceeds. The reaction is exothermic, and oxygen is released as a result of the decomposition.
- Collect the oxygen gas in the inverted jar submerged in the water trough. The gas will displace the water, and the jar will fill with oxygen.
Observation
Oxygen gas is released during the decomposition of hydrogen peroxide in the presence of manganese dioxide. The evolution of oxygen can be confirmed by testing it with a glowing splint. When a glowing splint is introduced into the jar filled with oxygen, it relights, confirming the presence of oxygen gas.
Reaction Equation
The decomposition reaction of hydrogen peroxide is as follows:
2H₂O₂ → 2H₂O + O₂↑
The manganese dioxide (MnO₂) acts as a catalyst, speeding up the reaction without being consumed in the process.
Precautions
- Handle hydrogen peroxide carefully, as it is a strong oxidizing agent and can cause burns or irritations.
- Ensure the conical flask is clean to avoid contamination of the chemicals.
- Use a properly fitted delivery tube to prevent the escape of gas and to direct it into the water trough.
- Always check the setup for leaks before starting the experiment to ensure efficient gas collection.