Aim
To demonstrate the electrolysis of water and the production of hydrogen and oxygen gases, and to observe the process of splitting water molecules into their constituent elements using electrical energy.
Materials Required
- Water
- Sodium hydroxide (NaOH) or dilute sulfuric acid (H₂SO₄) to increase the conductivity of water
- Electrolysis apparatus (including two electrodes, a power supply, and a container)
- Power supply (such as a battery or DC source)
Image Reference
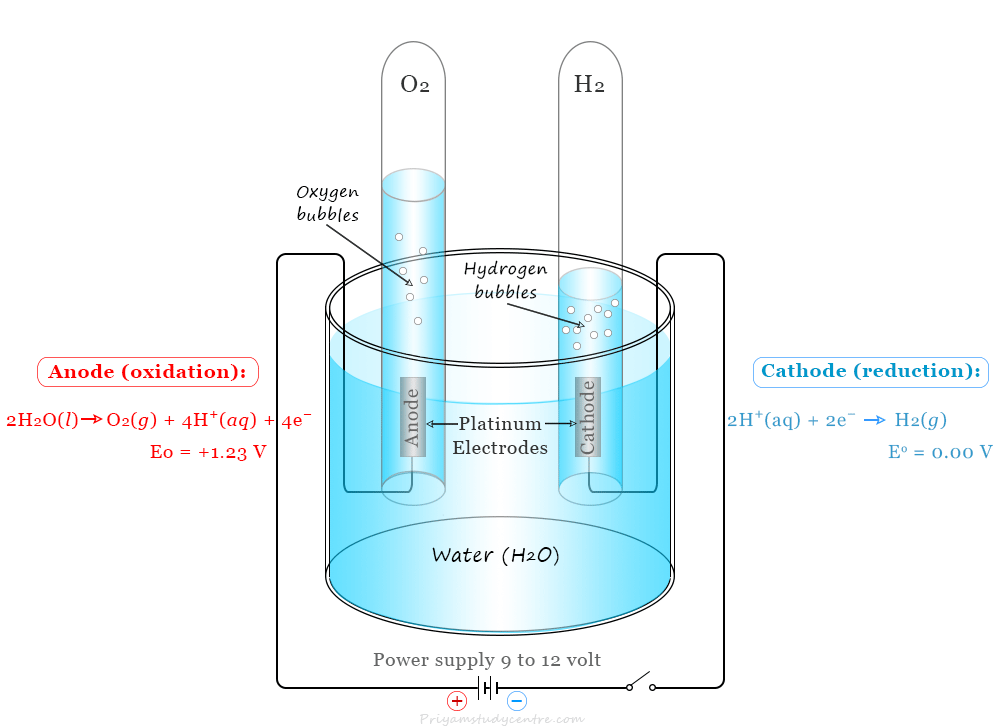
Procedure
- Fill the electrolysis apparatus with distilled water, and add a small amount of sodium hydroxide (NaOH) to increase the conductivity of the water. You can also use a small amount of sulfuric acid (H₂SO₄) for the same purpose.
- Connect the electrolysis apparatus to a power supply. Place two electrodes (usually made of platinum or graphite) in the water, ensuring they do not touch each other.
- Pass a current through the solution. Observe the gas bubbles that form at both electrodes.
- Note the volume of gas produced at each electrode. The gas produced at the cathode (negative electrode) is hydrogen, and the gas at the anode (positive electrode) is oxygen.
- The ratio of hydrogen to oxygen produced is 2:1 by volume, according to the electrolysis of water.
Observation
During the electrolysis of water, hydrogen gas is produced at the cathode (negative electrode), and oxygen gas is produced at the anode (positive electrode). The volume of hydrogen gas is approximately twice that of oxygen gas, indicating the molecular composition of water (H₂O) as two parts hydrogen and one part oxygen.
Reaction Equation
The overall reaction for the electrolysis of water is:
2H₂O(l) → 2H₂(g) + O₂(g)
Precautions
- Ensure that the electrodes do not touch each other to avoid short circuits.
- Handle the power supply carefully to avoid electrical hazards.
- Do not use pure water for electrolysis as it has very low conductivity. A small amount of NaOH or sulfuric acid should be added to increase conductivity.
- Wear safety goggles and gloves while performing the experiment, as hydrogen gas is flammable and oxygen supports combustion.