Aim
To construct and study the working of an electrochemical cell, and understand the conversion of chemical energy into electrical energy through redox reactions.
Materials Required
- Copper electrodes (Cu)
- Zinc electrodes (Zn)
- Dilute sulfuric acid (H₂SO₄)
- Voltmeter
- Connecting wires
- Beakers or containers to hold the solutions
Image Reference
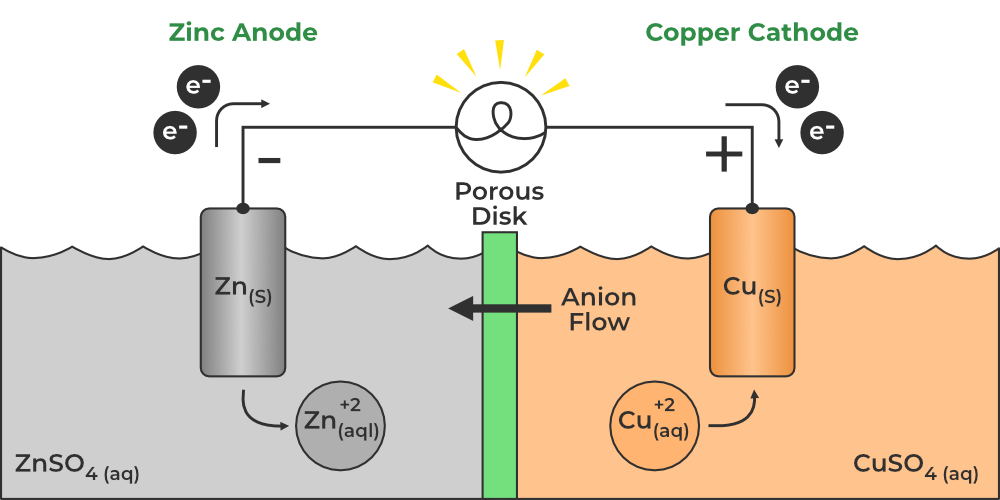
Procedure
- Prepare two beakers and fill one with dilute sulfuric acid (H₂SO₄), which will act as the electrolyte.
- Place a copper electrode in one beaker and a zinc electrode in the other beaker, ensuring both electrodes are immersed in the electrolyte.
- Connect the copper electrode to the positive terminal of the voltmeter and the zinc electrode to the negative terminal.
- Observe the voltmeter to check for any potential difference between the two electrodes, which demonstrates the electrochemical reaction.
- Note that the copper electrode undergoes reduction (gain of electrons) and the zinc electrode undergoes oxidation (loss of electrons), creating a flow of electrons through the wire, generating an electric current.
Observation
The electrochemical cell generates a potential difference (voltage), and the voltmeter registers a positive reading. The current flow is a result of the oxidation of zinc and the reduction of copper ions, demonstrating the principles of redox reactions.
Reaction Equations
- At the Zinc electrode (anode):
Zn(s) → Zn2+(aq) + 2e- - At the Copper electrode (cathode):
Cu2+(aq) + 2e- → Cu(s)
Precautions
- Ensure that the electrodes are clean and free from any contaminants before using them.
- Handle the sulfuric acid with care, as it is corrosive. Wear safety goggles and gloves during the experiment.
- Ensure that the electrodes are placed in the electrolyte with proper contact to avoid any short circuit.
- Do not touch the exposed ends of the electrodes connected to the voltmeter to avoid electric shocks.